Exploration of omega-side chain addition strategies for the syntheses of isocarbacyclin and 15R-16-(m-tolyl)-17,18,19,20-tetranorisocarbacyclin
Sheddan, Neil A.; Mulzer, Johann.
Organic & Biomolecular Chemistry (2006), 4(22), 4127-4130.
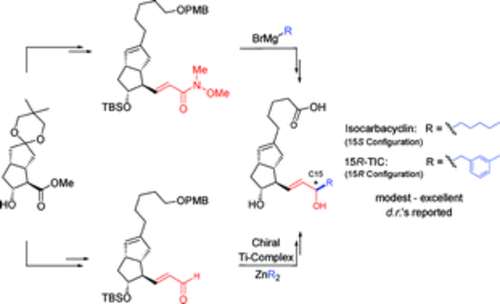
We describe alternative access to prostacyclin analogues by means of two omega-side chain addition strategies: Grignard reagent addition to an alpha,beta-unsaturated Weinreb amide, followed by diastereoselective reduction of the corresponding enone system, and implementation of Seebach's alkylation chemistry. These strategies have led to the syntheses of biologically active prostacyclin analogues isocarbacyclin and 15R-16-(m-tolyl)-17,18,19,20-tetranorisocarbacyclin (15R-TIC), with modest to excellent diastereoselectivity.
Johann Mulzer
Institute for Organic Chemistry
University of Vienna
Währingerstraße 38
A-1090 Vienna
Institute for Organic Chemistry
University of Vienna
Währingerstraße 38
A-1090 Vienna




