Total synthesis of efomycine M
Barth, Roland; Mulzer, Johann.
Angewandte Chemie, International Edition (2007), 46(30), 5791-5794.
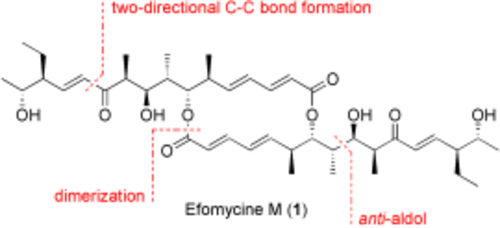
Two-directional synthesis: Key steps in the first total synthesis of the anti-inflammatory agent efomycine M (1) are a two-directional C11-C12 chain elongation and an early-stage dimerization reaction. The central stereopentad is synthesized by a highly stereoselective sequence consisting of an anti-aldol reaction and a diastereoselective reduction.
Johann Mulzer
Institute for Organic Chemistry
University of Vienna
Währingerstraße 38
A-1090 Vienna
Institute for Organic Chemistry
University of Vienna
Währingerstraße 38
A-1090 Vienna




