Two-directional total synthesis of efomycine M and formal total synthesis of elaiolide
Barth, Roland; Mulzer, Johann.
Tetrahedron (2008), 64(21), 4718-4735.
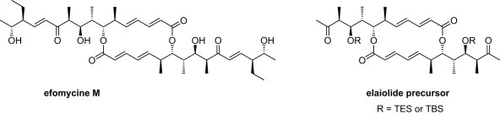
The anti-inflammatory agent efomycine M (1) has been synthesized from macrodilactone 38 and vinyliodide 42 by a two-directional total synthesis in 17 steps over the longest linear sequence with an overall yield of 7%. The C2-symmetric macrodiolide 38 has been prepared by Yamaguchi macrolactonization of seco-acid 26. The central stereopentad of 1 was obtained by a highly efficient anti-aldol reaction followed by a diastereoselective ketone reduction. Additionally, we have completed a formal total synthesis of elaiolide (3) by converting macrodiolide 37 into Paterson's methylketone 13.
Johann Mulzer
Institute for Organic Chemistry
University of Vienna
Währingerstraße 38
A-1090 Vienna
Institute for Organic Chemistry
University of Vienna
Währingerstraße 38
A-1090 Vienna




