Stereocontrolled Synthesis of the Tetracyclic Core Framework of (-)-Lemonomycin
Peter Siengalewicz, Lothar Brecker, Johann Mulzer*
Synlett (2008), (16), 2443-2446.
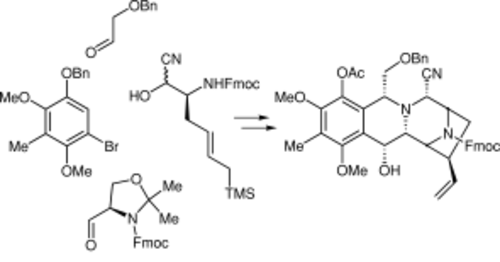
In a convergent approach, an advanced intermediate (2) in a projected total synthesis of the alkaloid (-)-lemonomycin (1) was prepared from readily available starting materials. The key transformations were a Pictet-Spengler cyclization, a Strecker-type amino alkylation, and an N-acyliminium cyclization.
Johann Mulzer
Institute for Organic Chemistry
University of Vienna
Währingerstraße 38
A-1090 Vienna
Institute for Organic Chemistry
University of Vienna
Währingerstraße 38
A-1090 Vienna




