Novel Protoilludane Lead Structure for Veterinary Antibiotics: Total Synthesis of Pasteurestins A and B and Assignment of Their Configurations
Marion Kögl, Lothar Brecker, Ralf Warrass, Johann Mulzer*
Eur. J. Org. Chem. 2008, 16, 2714–2730
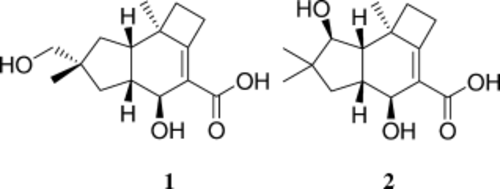
Two novel protoilludane sesquiterpenoids, named pasteurestins A and B (1 and 2), were disclosed in a recent patent. These compounds were reported to exhibit strong and selective activity against some Mannheimia haemolytica strains, pathogen causatives for bovine respiratory disease. These properties qualified 1 and 2 as potential lead structures for new veterinary antibiotics; however, neither the absolute nor the relative configurations had been determined, nor were the compounds available any longer. We thus developed total syntheses of 1 and 2 and clarified their structures and their biological profiles. Key steps were two [2+2+2] CpCo(CO)2-mediated Vollhardt cycloadditions in both syntheses, and a tin-mediated asymmetric Reformatsky-type condensation in the synthesis of 2 with a temperature-dependent product distribution.
Institute for Organic Chemistry
University of Vienna
Währingerstraße 38
A-1090 Vienna