Synthesis and biological evaluation of a des-dihydropyran laulimalide analog
Gollner, Andreas; Altmann, Karl-Heinz; Gertsch, Juerg; Mulzer, Johann.
Tetrahedron Letters (2009), 50(42), 5790-5792.
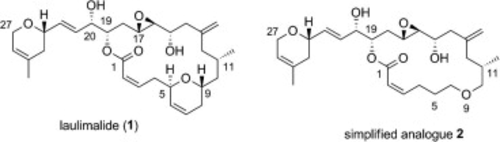
The preparation of a novel simplified Laulimalide analog via a highly convergent and efficient route and its biological evaluation are presented. The outlined route enables the synthesis of C5–C9 modified analog 2 and uses Julia–Kocienski olefination for fragment assembly and a regioselective Yamaguchi macrolactonization for ring closure. This strategy should be suitable for the generation of various new C5–C9 des-dihydropyran laulimalide derivatives for further SAR studies.
Johann Mulzer
Institute for Organic Chemistry
University of Vienna
Währingerstraße 38
A-1090 Vienna
Institute for Organic Chemistry
University of Vienna
Währingerstraße 38
A-1090 Vienna




