The Laulimalide Family: Total Synthesis and Biological Evaluation of Neolaulimalide, Isolaulimalide, Laulimalide and a Nonnatural Analogue
Gollner, Andreas; Altmann, Karl-Heinz; Gertsch, Juerg; Mulzer, Johann.
Chemistry-A European Journal (2009), 15(24), 5979-5997, S5979/1-S5979/81.
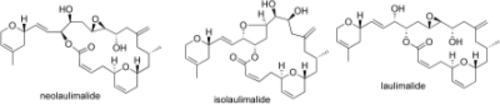
A sensitive family: The first total synthesis of the antitumor agents neolaulimalide and isolaulimalide as well as a highly efficient route to laulimalide is described. A Kulinkovich reaction followed by a cyclopropyl-allyl rearrangement is used to install the exo-methylene group. The cytotoxicity of neolaulimalide could be confirmed for the first time since its original isolation and it could be shown that it induces tubulin polymerization as efficiently as laulimalide.
Johann Mulzer
Institute for Organic Chemistry
University of Vienna
Währingerstraße 38
A-1090 Vienna
Institute for Organic Chemistry
University of Vienna
Währingerstraße 38
A-1090 Vienna




