Synthetic efforts towards the synthesis of the complex diterpene providencin
Gaich, Tanja; Weinstabl, Harald; Mulzer, Johann.
Synlett (2009), (9), 1357-1366.
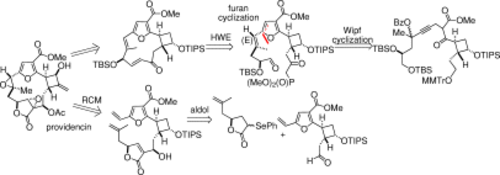
Providencin is a novel, highly oxygenated marine furanocembranolide featuring a cyclobutane ring and a highly strained 7,8-trans-epoxide. Various approaches to the total synthesis of this compound are reported. The cyclobutane moiety is generated via [2+2] cycloaddition and the furan ring is constructed via a Wipf palladium-catalyzed alkynone cyclization. The macrocyclic ring is closed via a Horner-Wadsworth-Emmons olefination or ring-closing metathesis. The latter reaction, however, produces the undesired 7,8-Z-olefin exclusively, and the conversion into the E-isomer has been, thus far, unsuccessful.
Johann Mulzer
Institute for Organic Chemistry
University of Vienna
Währingerstraße 38
A-1090 Vienna
Institute for Organic Chemistry
University of Vienna
Währingerstraße 38
A-1090 Vienna