Synthesis of (Z)-Trisubstituted Olefins by Decarboxylative Grob-Type Fragmentations: Epothilone D, Discodermolide, and Peloruside A
Prantz, Kathrin; Mulzer, Johann
Chemistry--A European Journal (2010), 16(2), 485-506, S485/1-S485/167
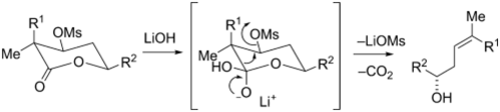
Methyl-branched (Z)-trisubstituted olefins are found in many polyketides with interesting biological activity, such as epothilone D (1), discodermolide (3), and peloruside A (2). Despite the employment of numerous different strategies, this motif has often been the weak point in total synthesis. Thus, we present a novel hydroxide- induced Grob-type fragmentation as an easy access to trisubstituted olefins. In our case, beta-mesyloxy gamma-lactones with three stereogenic centers were chosen whose fragmentation underlies a high stereoelectronic control. Major challenges in the syntheses were the installation of quaternary stereocenters, achieved by enzymatic desymmetrization of meso-diesters and by aluminium-promoted stereoselective rearrangement of chiral epoxides, respectively. Different aldol strategies were developed for the formation of the fragmentation precursors. Additionally a short survey about nucleophilic additions to aldehydes with quaternary alpha-centers is presented.
Institute for Organic Chemistry
University of Vienna
Währingerstraße 38
A-1090 Vienna




