Ring-Closing Metathesis and Photo-Fries Reaction for the Construction of the Ansamycin Antibiotic Kendomycin: Development of a Protecting Group Free Oxidative Endgame
Thomas Magauer, Harry J. Martin, Dr., Johann Mulzer, Prof.
Chemistry--A European Journal (2010), 16(2), 507-519
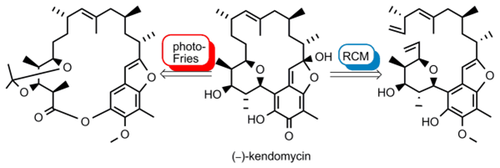
Two convergent total syntheses of the ansa-polyketide (-)-kendomycin (1) are described. The syntheses benefit from the use of readily available and cheap starting materials. Highly complex diastereoselective Claisen-Ireland rearrangements were used to introduce the (E)-double bond and the C16-Me group. The ring closure of the strained ansa macrocycle was achieved by ring-closing metathesis and a highly efficient combination of macrolactonization and photo-Fries reaction. A protecting group free endgame via an unstable o-quinone is presented. Additionally some unsuccessful synthetic efforts towards the total synthesis of 1 are described.
Institute for Organic Chemistry
University of Vienna
Währingerstraße 38
A-1090 Vienna