Efficient and Scalable One-Pot Synthesis of 2,4-Dienols from Cycloalkenones: Optimized Total Synthesis of Valerenic Acid
Juergen Ramharter and Johann Mulzer
<cite>Org. Lett., </cite>2011, 13 (19), pp 5310–5313
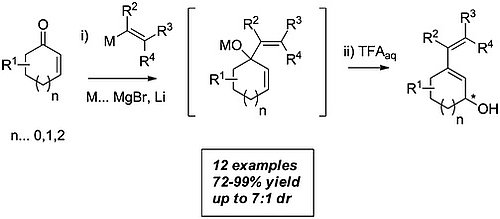
A mild and selective one-pot procedure to provide 2,4-dienols from simple cycloalkenones in high yields is described. This transformation is based on the in situ formation of acid-labile allylic alcohols, which on treatment with trifluoroacetic acid undergo a formal [1,3]-hydroxy migration to form diastereo- and enantiomerically enriched 2,4-dienols. The usefulness of this protocol is demonstrated in a short synthesis of valerenic acid.
Johann Mulzer
Institute for Organic Chemistry
University of Vienna
Währingerstraße 38
A-1090 Vienna
Institute for Organic Chemistry
University of Vienna
Währingerstraße 38
A-1090 Vienna




